Carbon and its Compound :-
CBSE Class 10 Science Chapter 4 Carbon and its Compounds Notes || Notes of Ch 4 carbon and its Compounds || Carbon and its Compounds Notes ||
Carbon and its Compound PDF :-
NOTES
Organic chemistry is that
branch of chemistry that deals with the study of carbon and its compounds.
Carbon is so versatile in nature that organic chemistry forms a separate branch of chemistry which deals mainly with carbon and its compounds.
Introduction to Carbon
- The Element Carbon is Non-Metal - Its Symbol is C.
- Carbon is a Versatile element the percentage Carbon in earth’s crust has only 0.02% carbon in the form of minerals (like carbonates, hydrogen carbonates ,coal and petroleum) and the atmosphere has 0.03% of carbon dioxide.
- All the living things, Plants and animals are made up of Carbon-based Compounds.
Carbon always form Covalent Bonds :-
Atomic number of Carbon is 6.
Electronic configuration :-
K L
C (6) 2 4
As suggested by its Configuration, it belongs to
second period and fourteen group.
Its valence electrons are 4.
Its valency is also 4.
How
Carbon attain Noble Gas Configuration ?
The atomic number of Carbon is 6 and its electronic Configuration is 2,4 to attain a noble gas configuration it can
- Gain 4 electrons But it would be difficult for nucleus to hold 4 extra electrons and is highly energy requiring process
- Lose 4 electrons But it would require a large amount of energy to remove 4 electrons.
- It is difficult thus for an atom of carbon to either gain or Lose electrons.
- Carbon attains the noble gas configuration by sharing its valence electrons with other atoms. Atoms of other elements Like hydrogen, Oxygen, Nitrogen, Chlorine also show sharing of Valence electrons.
- The bond formed by Sharing of electrons between same or different atoms is Covalent bond.
- Formation of H2 , O2 and N2 is shown as below



Physical Properties of Covalent Compounds
(a) Covalent Compounds have low melting and boiling points as they have
weak intermolecular Force.
(b) They are generally poor Conductor of electricity as electrons are shared between atoms and no Charged particles are formed.
Allotropes of Carbon :
(i) Diamond
(ii) Graphite
(iii) Fullerenes
These allotropes have same Chemical Properties. Difference between
diamond and graphite.
|
Diamond |
Graphite |
|
It is hardest natural substance |
It is Soft |
|
It is bad conductor of electricity but good conductor of heat. |
It is good
conductor of both electricity and heat. |
|
It is transparent |
It is Opaque |
Versatile Nature of Carbon
The two Characteristic properties of Carbon element which lead to the
formation of large number of compounds:
1. Catenation: It is the self
linking property of carbon i.e. it links with its own kind of atoms, giving
rise to long chains that are branched or ringed or can be straight.
Carbon
can link with carbon atoms by means of covalent bonds to form long chains ,
branched chains and closed ring Compound.
Carbon
atoms may linked by single , double or triple bonds.
E.g. : Silicon Forms compound with hydrogen up to seven or eight atoms of Silicon.


2. Tetravalency:- Carbon
has 4 electrons in its valence shell that it can easily loose, gain or share
with other atoms to attain stability.
Carbon
atom is capable of bonding with atoms of oxygen ,
hydrogen
, nitrogen , Sulphur , Chlorine and other elements.
The
smaller size of carbon atoms enables nucleus to hold the shared pair of
electrons strongly , thus carbon compounds are stable in general.
Hydrocarbons
There are two types of hydrocarbons –
|
General Formula |
Example |
ALKANE (CnH2n+2) |
CH4 , C2H6 , C3H8 , C4H10,C5H12 |
ALKENE (CnH2n) |
C2H4 , C3H6 , C4H8 , C5H10, C6H12 |
ALKYNE (CnH2n-2) |
C2H2 , C3H4 , C4H6 , C5H8, C6H10 |
The names , Molecular formulae and saturated formulae of saturated
hydrocarbons(alkanes) are given below:





Structural Isomers:- These are
the Compounds having Identical molecular formula but different structure are
called Structural isomers .
Heteroatom and Functional Groups :-
In hydrocarbons chain, one or more hydrogen atom is replaced by
other atoms in accordance With their valancies. The element that replaces hydrogen is called
a heteroatom.
These heteroatom and the group containing them impact
chemical properties to the Compound and hence are called Functional
groups.
Homologous Series :-
- It is
Series of Compounds in which the some Functional group substitutes for the
hydrogen in a carbon chain.
- E.g.
, Alcohols - CH3OH , C2H5OH, C3H7OH, C4H9OH
- Have Same general Formula.
- Any two homologous differ by – CH2 group and difference in molecular mass is 14 unit of mass.
- Have same chemical properties but show gradual change in physics properties.
- Melting point and boiling point increase with Increasing molecular mass.
Nomenclature of Carbon Compounds :-
(i) Identify the number of carbon atoms in Compounds.
(ii) Functional group is indicated by suffix or prefix.



CHEMICAL PROPERTIES OF CARBON COMPOUNDS:-
(a) Combustion:
- Carbon Compounds generally burn (Oxidize) in air to produce carbon dioxide and water and release heat and light energy.
CH4 + 02 ➡ C02 + H20 + heat and Light
- Saturated
hydrocarbon burns generally with a blue
flame in good supply of air and with a yellow sooty flame in limited supply of
air.
- sooty flame is seen when unsaturated hydrocarbons are burnt.
- Burning of coal and petroleum emits oxides of Sulphur and nitrogen which are responsible for acid rain.
(b) Oxidation
Alcohols can be converted to carboxylic acids by oxidizing them
using alkaline Potassium Permangnate
(KMnO4) or acidic potassium dichromate. (they add oxygen to the reactant thus are
called Oxidizing agents).
(c) Addition Reaction :
- Hydrogen is
added Unsaturated hydrocarbons in presence of catalysts
palladium or nickel Platinum as Catalyst.
- Vegetable oils are Converted into vegetable ghee using this process.
- Saturated Fatty acids are harmful for health and oils with unsaturated fatty acids should be used for cooking.
(d) Substitution Reaction :
In saturated hydrocarbons , the hydrogen attached to carbon
can be replaced by another or group if atoms in presence of sunlight.
IMPORTANT CARBON COMPOUNDS –
ETHANOL
AND ETHANOIC ACID
Physical Properties of Ethanol
- Colourless , Pleasant smell and burning taste.
- Soluble in water.
- Volatile liquid with low boiling point of 351K.
- Neutral Compound.
(i) Reaction With
Sodium :
Alcohols react with sodium leading to the
evolution of hydrogen (Burn with Pop Sound). With ethanol, the other product is
sodium ethoxide.
(ii) Dehydration
Reaction to give unsaturated hydrocarbon:
Heating ethanol at 443 K with excess concentrated sulphuric acid results in the
dehydration of ethanol to give ethene –

The concentrated sulphuric acid can be regarded as a dehydrating agent which removes water from ethanol.
Physical Properties of Ethanoic Acid
- Colourless
liquid having sour taste and have smell of vinegar.
- Boiling Point is 391 K.
- When Pure CH3COOH is freezed , It forms colurless ice like solid. So it is called glacial acetic acid.
Chemical Properties
Reactions of ethanoic acid:
(i) Esterification
reaction:
Esters are most commonly formed by reaction of an acid and an alcohol. Ethanoic
acid reacts with absolute ethanol in the presence of an acid catalyst to give
an ester –

Esters are sweet-smelling substances. These
are used in making perfumes and as flavouring agents. Esters react in the
presence of an acid or a base to give back the alcohol and carboxylic acid.
This reaction is known as saponification because it is used in the preparation
of soap.

(ii) Reaction with
base:
ethanoic acid reacts with a base such as
sodium hydroxide to give a salt (sodium ethanoate or commonly called sodium
acetate) and water:
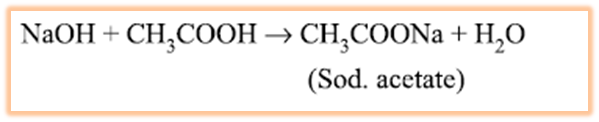
(iii) Reaction with carbonates and hydrogen Carbonates :
Ethanoic acid reacts with carbonates and hydrogen
carbonates to give rise to a salt, carbon dioxide and water. The salt produced
is commonly called sodium acetate.


SOAPS AND DETERGENTS
- Soap is Sodium and Potassium Salt of carboxylic acids with Long Chain.
- E.g. (C15H31COOH-Palmitic Acid) (C17H35COOH-Stearic Acid)
- Soaps are effective with soft water only and ineffective with hard water.
- Detergents are ammonium or sulphonate salts of hydrocarbons with long chain , they are effective with both soft as well as hard water.
Soap molecule has :
(i) Ionic (hydrophilic) Part.
(ii) Long hydrocarbon chain (hydrophobic) part.



Head is made up of ionic part that is water loving (hydrophilic)
and tail is made up of non-ionic part that is water repelling (hydrophobic).
Cleaning action of Soaps
:

- Most dirt is oily in nature and the hydrophobic end attaches itself with dirt and the ionic end is surrounded with molecules of water. This result information of a radial structure called micelles.
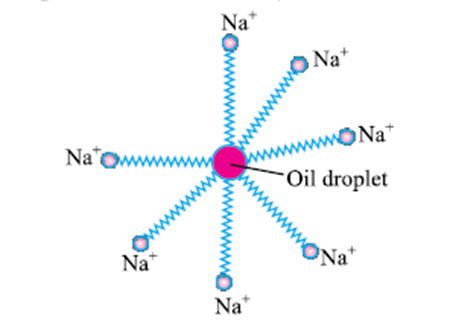
- An emulsion is thus formed by Soap molecule , the cloth needs to be mechanically agitated to remove the dirt particles from the Cloth.
- Scum : The magnesium and calcium salt present in hard water react with soap molecule to form insoluble product called scum. This scum create difficulty in cleansing action.
- By use of detergent, insoluble scum is not formed with hard water and cloths get cleaned Effectively.
Difference
between Soap and Detergent:-
|
Soap |
Detergent |
|
sodium salt of carboxylic
acid |
sodium salt of sulphonic
acid |
|
less soluble in water |
more soluble in water |
|
less powerful cleansing
agent |
more powerful cleansing
agent |
|
required in more amount |
required in less amount |
|
Biodegradable |
Non-Biodegradable |
Soaps are molecules in which the two ends have differing properties, one is hydrophilic, that is, it dissolves in water, while the other end is hydrophobic, that is, it dissolves in hydrocarbons. When soap is at the surface of water, the hydrophobic ‘tail’ of soap will not be soluble in water and the soap will align along the surface of water with the ionic end in water and the hydrocarbon ‘tail’ protruding out of water. Inside water,




these molecules have a unique orientation that keeps the hydrocarbon portion out of the water. This is achieved by forming clusters of molecules in which the hydrophobic tails are in the interior of the cluster and the ionic ends are on the surface of the cluster. This formation is called a micelle. Soap in the form of a micelle is able to clean, since the oily dirt will be collected in the Centre of the micelle. The micelles stay in solution as a colloid and will not come together to precipitate because of ion-ion repulsion. Thus, the dirt suspended in the micelles is also easily rinsed away. The soap micelles are large enough to scatter light. Hence a soap solution appears cloudy.






















0 Comments
Please do not enter any spam link in the comment box.